Therefore, not solely do valence electrons within an atom really feel the nucleus’ pull, however so do the valence electrons outside of an atom which would possibly be close enough to it. Since an atom desires a whole valence shell, so as to be as secure as a noble fuel, it freely attracts these valence electrons. But the valence electron of another atom also feels a pull because how many electrons does niobium have of the effective charge of its own nucleus. Therefore, the 2 atoms should share their electrons. The valency is zero as every noble gas’s outermost shell is stuffed hence they do not lose or achieve any electron. By the end of the article, students will know the means to calculate the valence electrons of oxygen and different components.
Hence, the group represents the variety of electrons present within the valence shell. Valence electrons, in easy words, are the electrons revolving continuously within the outermost shell or orbit of an atom. The outermost shell or the valence shell is the shell having the very best energy. Hence, the electrons present within the valence shell possess the best vitality compared to the electrons present within the inside orbits. As a end result, they could be attracted as much or extra by the nucleus of one other atom as by their nucleus.
Life Historical Past Of Silk Moth: Introduction, Varied Stages, Processing Of Silk
According to the Bohr-Bury system, an atom’s outermost shell can hold up to eight electrons. Furthermore, atoms with a totally stuffed outermost shell have little chemical exercise, implying that their valency is zero. It also indicates that they’re inactive substances. Helium has two electrons in its outermost shell, while the other inert components have atoms with eight electrons in their outermost shells. An octet is defined as an outermost shell with eight electrons.
- Do not want to achieve or lose electrons to achieve an octet.
- Similarly, Group 3 – Group eight are the place the $p$ orbital is being filled up.
- The bonds within the compounds these elements type due to this fact become much less ionic .
The variety of most valence electrons within the elements of this block is eight and the number of minimum valence electrons is three. The electron configuration of the p-block components reveals that the electron configuration ends in a p-orbital. Therefore, these components are known as p-block parts.
We will also learn how to tell how many valence electrons a component has. If every atom doesn’t have an octet configuration after the lone pairs have been allotted, a double or triple bond should be drawn to fulfill the octet valency of each atom. Each atom within the molecule now has a lone pair of electrons assigned to it. The most electronegative atoms are often assigned the lone pairs first. In the internal shell of a transition metallic, a valence electron can exist.
Module Three Valence Electrons
The outermost orbit of an atom accommodates most of eight electrons. But earlier than that, just take a look at the concept of valence electrons. There are many more labeled Periodic Table of valence electrons given below.
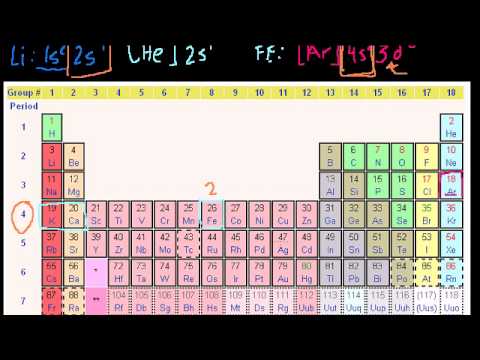
This activity can stand alone or be used with the attached worksheet and/or create different worksheets when discussing atom radius or ion radius… This activity allows students to make a software of models to help them visualize concepts of the periodic desk. Students will discover tendencies of ion expenses with relation to the periodic table. The electron was the first subatomic particle to be found.
Tips On How To Calculate Valence Electrons In A Molecule Or Polyatomic Ion
You can also get the printable Periodic desk with valence electrons, from this text solely. • Valence electrons are the outermost electron in an electron configuration. The octaves of these elements are full aside from helium. For instance, the atomic number of argon is eighteen. That is, the argon atom has a total of eighteen electrons. These parts don’t simply take part in any chemical reaction.
